Welcome to the Urbán lab
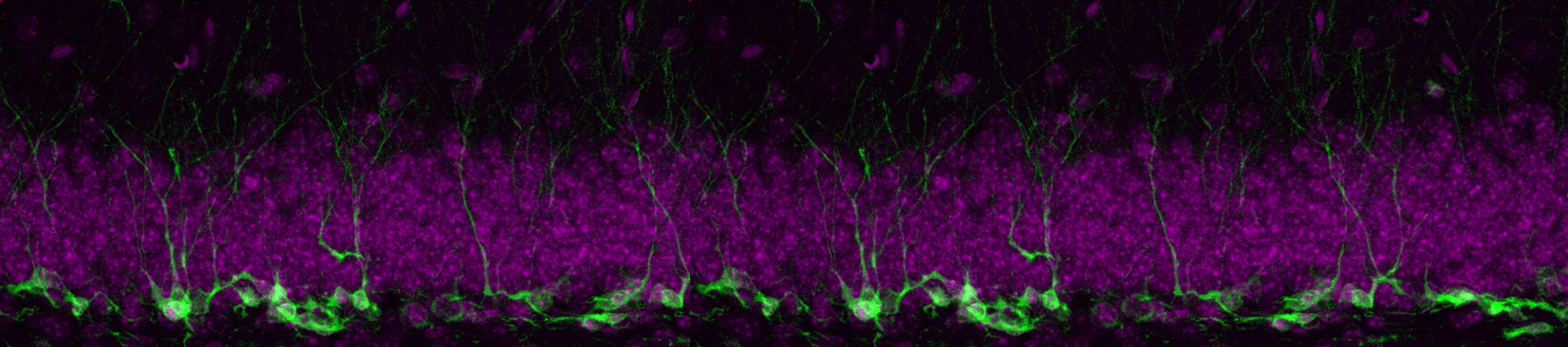
Stem cells are present in adult tissues and are essential for homeostasis and repair. In the brain of most mammals, including humans, adult neural stem cells (NSCs) persist in specific locations, like the dentate gyrus in the hippocampus. New neurons are generated in the dentate gyrus throughout life, which integrate into the existing hippocampal circuitry to modulate mood and memory. One key feature of adult NSCs is that, at any given time, most of them are in an inactive state - also called quiescence - until receiving the right activation stimuli. They also have a limited ability to self-renew, which means that their activation is tightly coupled with exhaustion. In fact, stem cell numbers rapidly decline with age and the subsequent loss of newborn neurons has been linked to symptoms characteristic of neural disorders such as dementia and depression.
Mission
Our research is directed towards elucidating the mechanisms driving the transition of NSCs between active and quiescent states, which ultimately will allow us to devise strategies to prevent their exhaustion during ageing. For this, we use and develop tools to manipulate adult neural stem cells in vivo and in vitro.

This graphical abstract was created by our research assistant Amarbayasgalan (Mara) Davaatseren
Selected Publications
Austin, SHL., Gabarró-Solanas, R., Rigo, P (...) Guillemot, F., Urbán, N. (2021). Wnt/β-catenin signalling is dispensable for adult neural stem cell homeostasis and activation. Development. 148(20)
Urbán, N., Cheung, TH. (2021). Stem cell quiescence: the challenging path to activation. Development. 148(3)
Harris, L., Rigo, P., Stiehl, T (...) Marciniak-Czochra, A., Guillemot, F. (2021). Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell. 28(5):863-876.e6
Urbán, N., Blomfield, IM., Guillemot, F. (2019). Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron. 104(5):834-848
Blomfield, IM., Rocamonde, B., Masdeu, MDM (...) Guillemot, F., Urbán, N. (2019). Id4 promotes the elimination of the pro-activation factor Ascl1 to maintain quiescence of adult hippocampal stem cells. Elife. 8
Urbán, N., van den Berg, DL., Forget, A (...) Ayrault, O., Guillemot, F. (2016). Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science. 353(6296):292-5
Andersen, J., Urbán, N., Achimastou, A (...) Nakafuku, M., Guillemot, F. (2014). A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 83(5):1085-97
Urbán, N., Guillemot, F. (2014). Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 8:396