Life depends on the constant renewal of faulty or damaged cellular components. Loss of this ability in animals leads to the accumulation of toxic waste products, which underlie diverse neurodegenerative and metabolic diseases. Similarly, plant cells must recycle worn out parts for optimal growth.
One of the major recycling pathways in the cell is autophagy. The cell labels damaged or faulty components with specific signals, which are then recognized by selective autophagy receptors. These receptors recruit the cargo into transport vesicles that deliver their cargo to recycling compartments. Identifying these receptors will help us understand how cells efficiently recycle these damaged components.
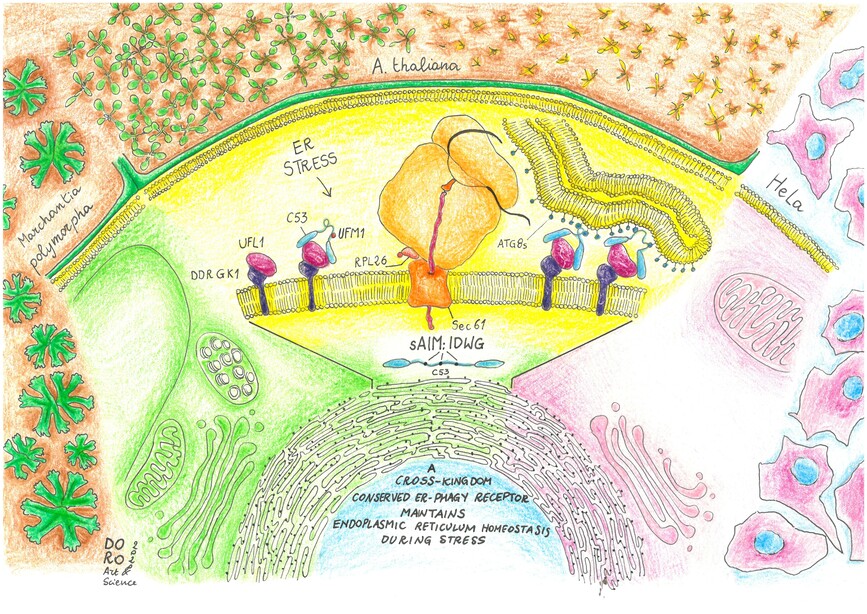
In this new work, the Dagdas lab identified new selective autophagy receptors that mediate recycling of the endoplasmic reticulum, the protein synthesis and folding hub of the cell. Protein synthesis and folding are both error-prone processes and several quality control pathways, including autophagy, help recycle faulty byproducts when errors occur.
They identified a modular protein, C53, which is conserved across all multicellular eukaryotes. Using both model plants (Arabidopsis thaliana and Marchantia polymorpha) as well as human HeLa cells, they found that C53 localizes to transport vesicles during endoplasmic reticulum stress. C53 interacts with the autophagy pathway via novel motifs located in a highly flexible region. By testing multiple mutants and model substrates, they showed that C53 is only activated by a very specific stimulus: faulty translation products that cause ribosomes attached to the endoplasmic reticulum to stall. As a result, c53 mutants of Arabidopsis thaliana and Marchantia polymorpha are highly sensitive to endoplasmic reticulum stress. Its high degree of conservation, new interaction motifs, and very specific activation stimulus make C53 a unique endoplasmic reticulum recycling receptor.
These findings mark an important step forward in our knowledge of cellular quality control mechanisms that maintain a healthy endoplasmic reticulum. C53 and its associated proteins have been linked to various diseases, including cancer and viral infections, and future studies could help identify the molecular mechanisms behind these associations. Further understanding of C53 and endoplasmic reticulum quality control pathways could one day be beneficial for developing crops that are more resilient to stress.
This work was carried out by an international team with members from the Gregor Mendel Institute, Research Institute of Molecular Pathology, University of Vienna, Max Perutz Labs, and the University of Natural Resources and Life Sciences from Vienna Austria as well as the Chinese University of Hong Kong, Leibniz Institute of Plant Biochemistry and Science Halle Germany, IPK Gatersleben Germany, and the Medical Institute of Bioregulation Kyushu University Japan. Funding was provided by Vienna Science and Technology Fund, Austrian Science Fund, Austrian Academy of Sciences, European Research Councl, and the The Financial Supports for Young Scientists International Research Scholarship Fund.
Madlen S, Picchianti L, ..., Dagdas Y (2020) A cross-kingdom conserved ER-phagy receptor maintains endoplasmic reticulum homeostasis during stress. eLife:58396 preprint bioRxiv:995316.