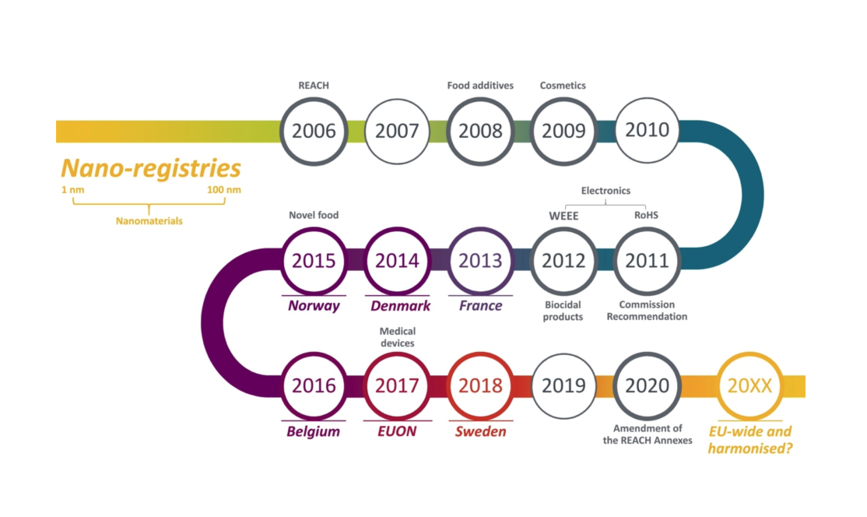
The regulation of nanomaterials is approached differently by the European Commission, the European Parliament and individual members like France, Belgium or Denmark. The Institute of Technology Assessment of the Austrian Academy of Sciences (ITA-ÖAW) has examined the various existing nano-registries within the EU and EEA. The conclusion: A harmonised EU-wide nano-registry would substantially improve the current heterogenous situation and reduce uncertainties regarding the safety of nanomaterials.
Vast body of EU-legislation not followed by all
Within the EU, high standards are set regarding human health and the environment according to the precautionary principle. There is a vast body of legislations on the EU-level explicitly mentioning nanomaterials. In addition to REACH legislation, nanomaterials are specifically addressed and explicitly mentioned within sector-specific regulations in the area of cosmetics, biocides, the food or electronics sector as well as in the regulation on medical devices. However, some countries have introduced national nanomaterial reporting schemes, which generates a heterogenous situation.
Risk-prevention should be on top
Although there are many commonalities between the national nanomaterial reporting schemes – e.g. they all follow the EC recommendation on the definition of nanomaterial, provide information on substances below the REACH threshold and operate via an online portal – they also have many differences. They differ in their scope, in their formats, in their information requirements, and in their exemptions. This heterogenous framework hampers comparability. Additionally, the evaluation reports are not published on a frequent basis and are only available in the respective official languages of the countries.
- give reliable quantitative input data for risk assessment (i.e. production volumes and use in products), which would provide overview of what is produced, processed and used for certain purposes and in what quantities,
- facilitate traceability of chemicals along the whole supply and value chain,
- create transparency,
- help to close knowledge gaps about market distribution, circularity of substance flows and environmental fate and, in effect, increase trust in the safety of nanomaterials and products and
- generally foster sustainable research and innovation instead of slowing it down. Therefore, the ITA believes that a harmonised EU-wide nano-registry would substantially improve the current heterogenous situation and reduce uncertainties regarding the safety of nanomaterials.